A Proven Non-Drug Hypertension Treatment

RESPeRATE safety and efficacy in lowering blood pressure were demonstrated repeatedly in 20 separate clinical research studies published in peer-reviewed medical journals [2-22]
RESPeRATE delivers a significant, all-day long, sustained reduction in blood pressure, when measured at the office[2-19], at home [3-7, 9, 12-13], and even with 24-hour Ambulatory Blood Pressure Monitor [4, 10, 18].

RESPeRATE’s clinical evidence won it the world’s only FDA clearance for non-drug treatment of hypertension.
Improved sleep was the only “side effect” reported by over 250,000 patients and doctors in the past 15 years.
“The overall evidence from clinical trials and meta-analyses suggests that device-guided slow breathing can significantly lower blood pressure.”
American Heart Association Scientific Statement Brook RC et al. 2013;61(6):1360-1383.
Learn More about AHA StatementPlease note: American Heart Association does not specifically recommend or endorse use of any products or brands.
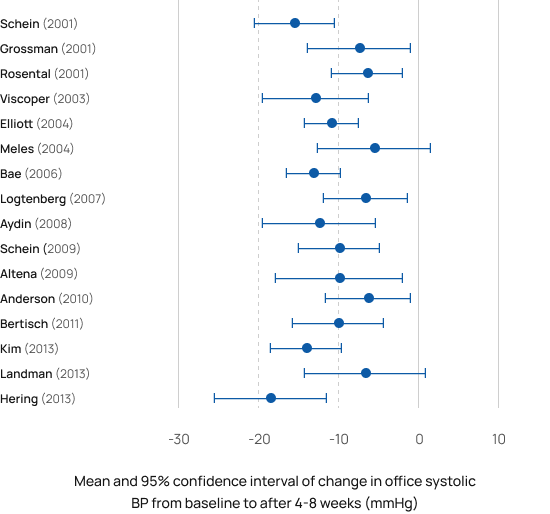
Found safe & effective in a wide range of patients
RESPeRATE was found safe and effective in populations ranging from healthy normotensive [28] to refractory hypertensives [5]. The studies performed in the US, Europe, Middle East, and the Far East also found it was effective in patients with comorbidities as diabetes [9, 11, 18], chronic kidney failure [19], gestational hypertension(i.e. pregnant woman ) [21] , pulmonary arterial hypertension [41,42], CHF [23, 32-39], COPD [40-41], PTSD [48-50] and more. Weighted average from the studies was 10/5 mmHg sustained office BP reduction in adults both with controlled and uncontrolled BP. BP reductions were independent of gender and medication status.
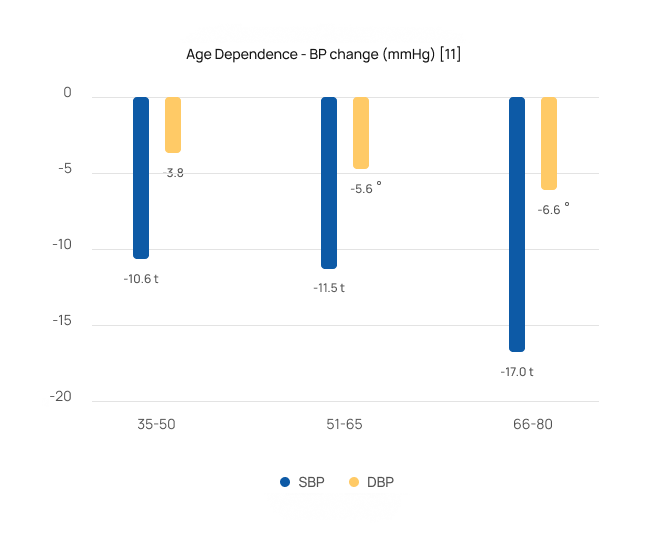
Safe & effective for the most severe patients
Studies [2-8,10-11] showed that patients over 65 saw greater BP reductions averaging 16/7 mmHg. They also demonstrated that patients with stage 2 hypertension (systolic BP>160 mmHg) saw larger reductions averaging 17/7 mmHg.
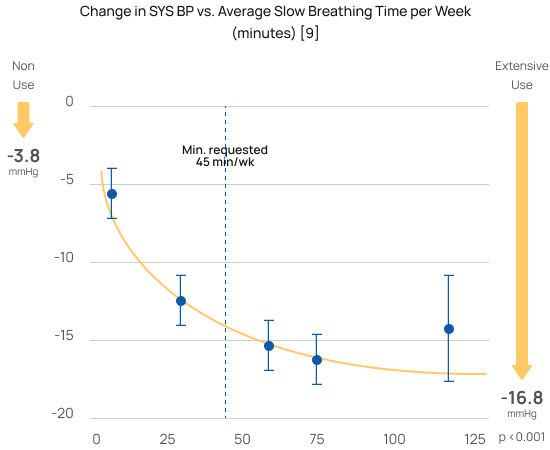
Greater reductions with greater use
The randomized controlled study [6] that won RESPeRATE it’s FDA-clearance for use without a prescription (OTC) demonstrated that slow breathing was the “active component” and a “dose-response.” Using RESPeRATE for only 45 minutes a week of slow breathing delivers significant blood pressure reductions. Using RESPeRATE more yields an even larger reduction.
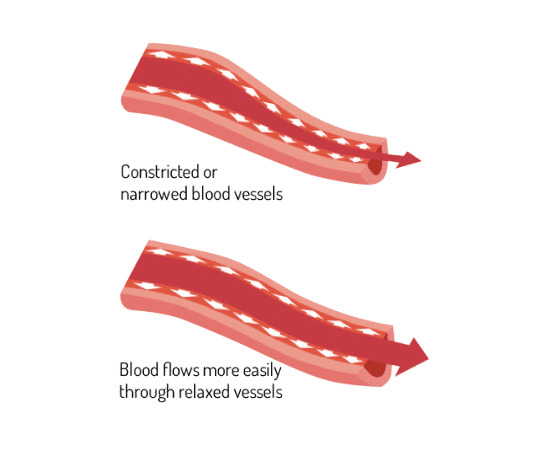
Proven to dilate blood vessels
Eight studies demonstrated the physiological mechanism of action behind RESPeRATE effects. See the scheme in the medical textbook HypertensionPrimer [24]. The studies demonstrated that RESPeRATE delivers an acute reduction of the sympathetic neural activity [22, 23, 25-27], resulting among other things in a sustained decrease in peripheral resistance and arterial stiffness [28-30].
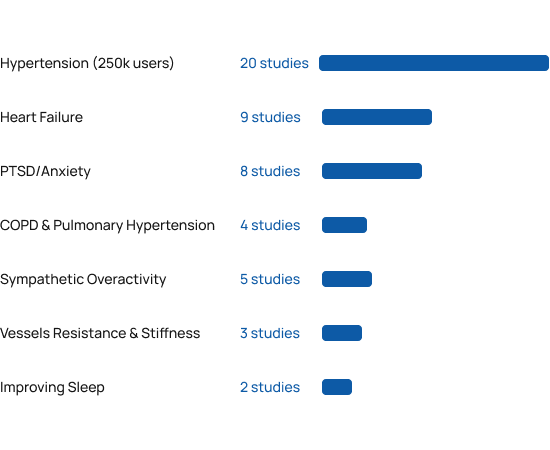
Benefits beyond blood pressure
Beyond the 20 clinical studies in hypertension, independent researchers around the world have published more than 30 additional clinical studies demonstrating the various cardiopulmonary and neurological benefits of RESPeRATE. Please note that RESPeRATE has been cleared for marketing only for lowering blood pressure and stress.
References
Hypertension
- Brook RC et al. A Scientific Statement From the American Heart AssociationBeyond Medications and Diet: Alternative Approaches to Lowering Blood Pressure.Hypertension. 2013;61(6):1360-1383
- Schein MH, Gavish B, Herz M, Rosner-Kahana D, Naveh P, Knishkowy B, Zlotnikov E, Ben-Zvi N, Melmed RN. Treating hypertension with a device that slows and regularises breathing: a randomised, double-blind controlled study. J Hum Hypertens. 2001;15:271-278.
- Grossman E, Grossman A, Schein MH, Zimlichman R, Gavish B. Breathing-control lowers blood pressure. J Hum Hypertens 2001;15:263–9.
- Rosenthal T, Alter A, Peleg E, Gavish B. Device guided breathing exercises reduceblood pressure: ambulatory and home measurements. Am J Hypertens2001;14:74–76.
- Viskoper R, Shapira I, Priluck R, Mindlin R, Chornia L, Laszt A, et al.Nonpharmacologic treatment of resistant hypertensives by device-guided slowbreathing exercises. Am J Hypertens 2003;16:484–487.
- Elliott WJ, Izzo JL Jr, White WB, Rosing DR, Snyder CS, Alter A, et al. Graded bloodpressure reduction in hypertensive outpatients associated with use of a device toassist with slow breathing. J Clin Hypertens (Greenwich) 2004;6:553–559.
- Meles E, Giannattasio C, Failla M, Gentile G, Capra A, Mancia G. Nonpharmacologictreatment of hypertension by respiratory exercise in the home setting. Am JHypertens 2004;17:370–4.
- Bae JH, Kim JH, Choe KH, Hong SP, Kim KS, Kim CH, Ko JK, Kim CH, Kim KS. Bloodpressure change following 8-week, 15-minute daily treatment with pacedbreathing guided by a device: a Korean multi-center study. J Clin Hypertens2006;8:79 [Korean with abstract in English in Korean Hyperten J 2006;1:19-23]
- Logtenberg SJ, Kleefstra N, Houweling ST, Groenier KH, Bilo HJ. Effect of device-guided breathing exercises on blood pressure in hypertensive patients with type 2 diabetes mellitus: a randomized controlled trial. J Hypertens 2007;25:241–6.
- Aydin L, Erdine S, Altintas L, Ucak S, Altuntas Y, Sisli Etfal. Device-guided paced breathing reduces blood pressure – ambulatory and office measurements. J Hyperten 2008; 26 (suppl 2): S371-S372 [abstract]
- Schein MH, Gavish B, Baevsky T, Kaufman M, Levine S, Nessing A, et al. Treating hypertension in type II diabetic patients with device-guided breathing: a randomized controlled trial. J Hum Hypertens 2009; 23:325–331.
- Altena MR, Kleefstra N, Logtenberg SJ, Groenier KH, Houweling ST, Bilo HJ. Effect of device-guided breathing exercises on blood pressure in patients with hypertension: a randomized controlled trial. Blood Press 2009;18:273–9
- Anderson DE, McNeely JD, Windham BG. Regular slow-breathing exercise effects on blood pressure and breathing patterns at rest. J Hum Hypertens 2010;24:807–813.
- Bertisch SM, Schomer A, Kelly EE, Baloa LA, Hueser LE, Pittman SD, et al. Device-guided paced respiration as an adjunctive therapy for hypertension in obstructive sleep apnea: a pilot feasibility study. Appl Psychophysiol Biofeedback 2011;36:173–1792
- Kim JH, Yoo BS, Lee SH, Yoon J, Choe KH, The Changes of Noninvasive Hemodynamic Parameters after Device-Guided Slow Breathing Exercise in Hypertensive Patients. J Korean Soc Hypertens 2013;19:55-62
- Landman GW, Drion I, van Hateren KJ, van Dijk PR, Logtenberg SJ, Lambert J, et al. Device-guided breathing as treatment for hypertension in type 2 diabetes mellitus: a randomized, double-blind, sham-controlled trial. JAMA Intern Med 2013;173:1346–1350.
- Hering D, Kucharska W, Kara T, Somers VK, Parati G, Narkiewicz K. Effects of acute and long-term slow breathing exercise on muscle sympathetic nerve activity in untreated male patients with hypertension. J Hypertens. 2013;31:739-746.
- Howorka K, Pumprla J, Tamm J, Schabmann A, Klomfar S, Kostineak E, et al. Effects of guided breathing on blood pressure and heart rate variability in hypertensive diabetic patients. Auton Neurosci 2013;179:131–137.
- Gusmão Josiane Lima de; Carminatte, Deise Aparecida. Device-guided slow breathing reduces the post hemodialysis blood pressure in hypertensive patients with chronic kidney disease. J Hypertension: September 2012 – p e141[Abstract]
- Wojcicki JM, Geissler JD, Stokes CW, Heyman MB, Tran CT. The use of the RESPeRATE device to lower blood pressure in inner city obese adolescents and children: a pilot feasibility study. High Blood Press Cardiovasc Prev. 2013 Jun;20(2):89-92.
- El-Bandrawy AM, Ghareeb HO. Effect of aerobic exercise versus device-guided breathing on gestational hypertension. Physiotherapy Quarterly. 2023;31(3):36-40. doi:10.5114/pq.2023.117436.
Lowering neural sympathetic activity
- Oneda B, Ortega KC, Gusmão JL, Araújo TG, Mion D Jr. Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens Res. 2010 Jul;33(7):708-712.
- Harada D, Asanoi H, Takagawa J, Ishise H, Ueno H, Oda Y et al. Slow and deep respiration suppresses steady-state sympathetic nerve activity in patients with chronic heart failure: from modeling to clinical application. American Journal of Physiology-Heart and Circulatory Physiology. 2014;307(8):H1159-H68
- Parati G et al, In Hypertension Primer Eds. Izzo et al.Lippincott Williams & Wilkins. 3rd Edition 2003, pp. 117-120.
- de Barros S, da Silva GV, de Gusmão JL, de Araujo TG, Mion D Jr. Reduction of sympathetic nervous activity with device-guided breathing. J Clin Hypertens (Greenwich). 2014 Aug;16(8):614-5.;
- de Barros S, da Silva GV, de Gusmão JL, de Araújo TG, de Souza DR, Cardoso CG Jr, Oneda B, Mion D Jr. Effects of long term device-guided slow breathing on sympathetic nervous activity in hypertensive patients: a randomized open-label clinical trial. Blood Press. 2017 Dec;26(6):359-365.
- Adler TE, Coovadia Y, Cirone D, Khemakhem ML, Usselman CW. Device-guided slow breathing reduces blood pressure and sympathetic activity in young normotensive individuals of both sexes. J Appl Physiol (1985). 2019 Oct 1;127(4):1042-1049.
Sustained decrease in peripheral resistance and arterial stiffness
- Gavish, Benjamin et al. “Effect of non-drug interventions on arterial properties determined from 24-h ambulatory blood pressure measurements.” Hypertension research : official journal of the Japanese Society of Hypertension vol. 34,11 (2011): 1233-8.
- Ovadia-Blechman Z, Gavish B, Levy-Aharoni D, Shashar D, Aharonson V. The coupling between peripheral microcirculation and slow breathing. Med Eng Phys. 2017 Jan;39:49-56.
- Faconti L, Farukh B, McNally R, Webb A, Chowienczyk P. Arterial Stiffness Can Be Modulated by Pressure-Independent Mechanisms in Hypertension. J Am Heart Assoc. 2019 Aug 6;8(15):e012601
- Bachler M, Sehnert W, Mikisek I, Wassertheurer S, Mengden T. Non-invasive quantification of the effect of device-guided slow breathing with direct feedback to the patient to reduce blood pressure. Physiol Meas. 2020 Nov 6;41(10)
CHF-COPD & Pulmonary Arterial Hypertension
- Parati G, Malfatto G, Boarin S, Branzi G, Caldara G, Giglio A, Bilo G, Ongaro G, Alter A, Gavish B, Mancia G. Device-Guided Paced Breathing in the Home Setting. on Exercise Capacity, Pulmonary and Ventricular Function in Patients With Chronic Heart Failure: A Pilot Study. Circulation: Heart Failure. 2008;1:178-183
- Ekman I, Kjellström B, Falk K, Norman J, Swedberg K. Impact of device-guided slow breathing on symptoms of chronic heart failure: a randomized, controlled feasibility study. Eur J Heart Fail. 2011;13:1000-1005.
- Bilo G, Revera M, Bussotti M, Bonacina D, Styczkiewicz K, Caldara G, Giglio A, Faini A, Giuliano A, Lombardi C, Kawecka-Jaszcz K, Mancia G, Agostoni P, Parati G. Effects of slow deep breathing at high altitude on oxygen saturation, pulmonary and systemic hemodynamics. PLoS One. 2012;7(11):e49074.
- Debicka-Dabrowska D, Lisi E, Drozdz T, Styczkiewicz K, Malfatto G, Salerno S, Bednarek A, Olszanecka A, Kielbasa G, Bilo G, Czarnecka, D, Kawecka-Jaszcz K, Parati G. Usefulness of slow breathing training in chronic heart failure. Study design and intermediate results. Journal of Hypertens 2015:33 e-Supplement1; e 462
- Drozdz T, Bilo G, Debicka-Dabrowska D, Klocek M, Malfatto G, Kielbasa G, Styczkiewicz K, Bednarek A, Czarnecka D, Parati G, Kawecka-Jaszcz K. Blood pressure changes in patients with chronic heart failure undergoing slow breathing training. Blood Press. 2016;25(1):4-10
- Kawecka-Jaszcz, Kalina & Bilo, Grzegorz & Drożdż, Tomasz & Dębicka-Dąbrowska, Dorota & Kiełbasa, Grzegorz & Malfatto, Gabriella & Styczkiewicz, Katarzyna & Lombardi, Carolina & Bednarek, Agnieszka & Salerno, Sabrina & Czarnecka, Danuta & Parati, Gianfranco. (2017). Effects of device-guided slow breathing training on exercise capacity, cardiac function and respiratory patterns during sleep in male and female patients with chronic heart failure. Polskie Archiwum Medycyny Wewnetrznej. 127. 10.20452/pamw.3890.
- Lachowska, K., Bellwon, J., Narkiewicz, K. et al. Long-term effects of device-guided slow breathing in stable heart failure patients with reduced ejection fraction. Clin Res Cardiol (2018)
- Lachowska K, Bellwon J, Moryś J, Gruchała M, Hering D. Slow breathing improves cardiovascular reactivity to mental stress and health-related quality of life in heart failure patients with reduced ejection fraction. Cardiol J. 2019 Jan 30. doi: 10.5603/CJ.a2019.0002 [Epub ahead of print]
- Lachowska K, Bellwon J, Moryś J, Gruchała M, Hering D. Slow breathing improves cardiovascular reactivity to mental stress and health-related quality of life in heart failure patients with reduced ejection fraction. Cardiol J. 2019 Jan 30. doi: 10.5603/CJ.a2019.0002 [Epub ahead of print]
- Wang J, Guo S, Zeng M, Yu P, Mo W. Observation of the curative effect of device-guided rehabilitation on respiratory function in stable patients with chronic obstructive pulmonary disease. Medicine (Baltimore). 2019.
PTSD/Anxiety/Stress
- de Jong MC, Boersma CH. Device-guided breathing as a possible tool to improve the outcome of exposure therapy.Mental Illness 2010;2:e6 [pp.25-27]
- Morarend QA, Spector ML, Dawson DV, Clark SH, Holmes DC. The use of a respiratory rate biofeedback device to reduce dental anxiety: an exploratory investigation. Appl Psychophysiol Biofeedback. 2011 Jun;36(2):63-70
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4253397/
- https://pubmed.ncbi.nlm.nih.gov/28283805/
- Wahbeh H, Goodrich E, Goy E, Oken BS. Mechanistic Pathways of Mindfulness Meditation in Combat Veterans With Posttraumatic Stress Disorder. J Clin Psychol. 2016 Apr;72(4):365-83.
- Fonkoue IT, Marvar PJ, Norrholm SD, Kankam ML, Li Y, DaCosta D, Rothbaum BO, Park J. Acute effects of device-guided slow breathing on sympathetic nerve activity and baroreflex sensitivity in posttraumatic stress disorder. Am J Physiol Heart Circ Physiol. 2018 Jul 1;315(1):H141-H149
- Fonkoue IT, Hu Y, Jones T, et al. Eight weeks of device-guided slow breathing decreases sympathetic nervous reactivity to stress in posttraumatic stress disorder. Am J Physiol Regul Integr Comp Physiol. 2020;319(4):R466-R475.
- Gabriely R, Tarrasch R, Velicki M, Ovadia-Blechman Z. The influence of mindfulness meditation on inattention and physiological markers of stress on students with learning disabilities and/or attention deficit hyperactivity disorder. Res Dev Disabil. 2020 May;100:103630. Res Dev Disabil. 2020 May;100:
- Ovadia-Blechman Zehava, Tarrasch Ricardo, Velicki Maria, Chalutz Ben-Gal Hila. Reducing Test Anxiety by Device-Guided Breathing: A Pilot Study. Frontiers in Psychology. 2022 vol 13
Other
- Huang AJ1, Phillips S, Schembri M, Vittinghoff E, Grady D. Device-guided slow-paced respiration for menopausal hot flushes: a randomized controlled trial. Obstet Gynecol.
- Anderson DE, McNeely JD, Windham BG. Device-guided slow-breathing effects on end-tidal CO(2) and heart-rate variability. Psychol Health Med. 2009 Dec;14(6):667-79
- Huang AJ1, Phillips S, Schembri M, Vittinghoff E, Grady D. Device-guided slow-paced respiration for menopausal hot flushes: a randomized controlled trial. Obstet Gynecol.
- Anderson DE, McNeely JD, Windham BG. Device-guided slow-breathing effects on end-tidal CO(2) and heart-rate variability. Psychol Health Med. 2009 Dec;14(6):667-79
- Huang AJ, Grady D, Mendes WB, Hernandez C, Schembri M, Subak LL. Controlled Trial of Device Guided Slow-Paced Respiration in Women with Overactive Bladder Syndrome. J Urol. 2019 Oct; 202(4): 787–794.
- Savoie MB, Lee KA, Subak LL, Hernandez C, Schembri M, Fung CH, Grady D, Huang AJ. Beyond the bladder: poor sleep in women with overactive bladder syndrome. Am J Obstet Gynecol. 2020 Jun;222(6):600.e1-600.e13.

Get Your RESPeRATE Now!
Risk-free Order: 60 day money back guarantee
A one-time payment of $349 .95 or pay in 4 interest-free installments of:
$87.49+ Free S&H
Pay 4 installments
Pay in 4 interest-free installments so you can spread the cost.
Add item(s) to your cart
Go to checkout and choose
Enter your debit or credit card information
Pay later in 4 installments. The first payment is taken when the order is processed and the remaining 3 are automatically taken every two weeks.
See payment terms. A higher initial payment may be required for some consumers. CA residents: Loans made or arranged pursuant to a California Financing Law license.
Get our FREE 10 Tips to Lower Blood Pressure Naturally
You’ll also receive our weekly newsletter.
AHA Scientific Statement: Beyond Medications and Diet: Alternative Approaches to Lowering Blood Pressure
Device-Guided Slow Breathing - Summary and Clinical Recommendations
See AHA full Statement“The overall evidence from clinical trials and meta-analyses suggests that device-guided slow breathing can significantly lower BP. There are no known contraindications to the use of the device, and no adverse effects have been noted.[104] Unfortunately, the device currently costs in excess of $200 in the United States; however, it has recently been included on the British National Health Service’s Drug Tariff (Part IX), which makes its cost reimbursable if prescribed by a physician. Specific recommendations for use are outlined elsewhere[110] and by the manufacturer (https://www.resperate.com). Another limitation is that device-guided slow breathing has not been directly compared with other forms of regulated breathing such as pranayama. Hence, it remains unknown whether paced slow breathing can be taught and effectively used to lower BP over the long term without the use of a device. According to trial evidence, 15-minute sessions of device-guided slow breathing need to be performed at least 3 to 4 times per week to reduce BP. It has been suggested that more frequent use may lead to greater BP lowering; however further evidence is required in this regard. There have been no trials longer than 8 to 9 weeks’ duration; hence, the efficacy of long term use is unclear. Longer and larger studies are also required to demonstrate the patient populations most likely to benefit from this technology.[88,89,110,111] The writing group conferred to device-guided breathing a Class IIA, Level of Evidence B recommendation for BP-lowering efficacy. Device-guided breathing is reasonable to perform in clinical practice to reduce BP. Should additional studies in larger and broader populations corroborate its effectiveness thus far demonstrated, it is conceivable that this technique may merit even stronger recommendations in the future.”
Please note: American Heart Association does not specifically recommend or endorse use of any products or brands.








 Download Brochure
Download Brochure